Overcoming the Transport Barriers of Brain Tumors
Improved penetration along with accurate prediction and mechanistic understanding of therapeutic agent delivery across the brain vessels and within the tumor are essential for the rational development of effective therapeutic strategies in brain cancer. Focused ultrasound in combination with microbubbles – tiny lipid or albumin bubbles that vibrate in response to ultrasound (i.e. acoustic cavitation) – injected into the circulation offers the unique ability to promote and control mass transport across the brain vessels and in the tumor core. While FUS in combination with microbubbles can lead to more than 4-fold increase in the delivery of a wide range of therapeutic agents, it remains unclear how the microbubble oscillations interact with the highly heterogeneous tumor microenvironment in order to achieve the reported increase in drug delivery.
To decipher the underlying microbubble-mediated mass transport mechanisms in the brain, our lab combines mathematical modeling with quantitative imaging to study and characterize the transvascular and interstitial transport in the brain with sub-cellular resolution. We have recently demonstrated, that 1) ultrasound leads to high transvascular mass transport and increased flow of interstitial fluid between tumor cells resulting in better penetration of two common types of chemotherapeutics in the tumor, 2) provided evidence of increased drug uptake by endothelial cells, and 3) identified optimal conditions for improved transport of therapeutics. For example, due to their very low diffusivity, the transport of large molecules is primarily mediated by convection. Our approach provided justification for the use of this technology with larger therapeutic agents, including antibodies (~15 nm in diam.) and nanoparticles (~20-60 nm in diam). Understanding how do the physicochemical properties of any given drug (e.g. molecular weight, cell binding affinity, etc.) influence drug transport in the brain during FUS in combination with microbubbles is a critical next step to attain effective therapy.
The establishment of the proposed theoretical framework and the insights gained from experimental characterization of the brain tumor microenvironment could facilitate the engineering of novel therapeutic strategies in the brain and enable the refinement of experimental and clinical protocols, which currently rely on intuition and time consuming and costly trial and error.
 |
 |
|
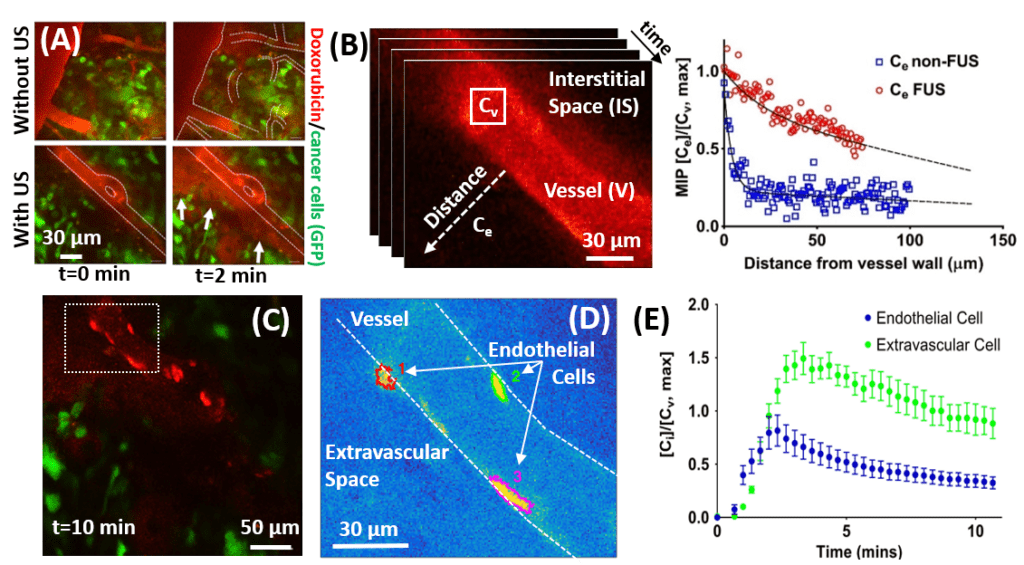
| In vivo quantification of drug transport in breast brain metastasis mouse model. (A) Intravital multiphoton microscopy of doxorubicin distribution in a brain cancer model with and without FUS with microbubbles meditated BBB disruption. (B) Quantification of doxorubicin extravassation (C)-(E) Quantification intracellular doxorubicin kinetics during microbubble-meditated BBB disruption. PNAS 2018 | Mathamatical modeling of transport in artifical vascular netwoprks that simulate heterogenous perfusion (percolation model). The arrows and different colors indicate the interstitial velocity, the transvascular pressure difference (thresholeded to highlight postive and negative regions), the transvascular drug flux, and the interstitial drug concentration. PNAS 2018 |
Controlled Release and Delivery of Therapeutics
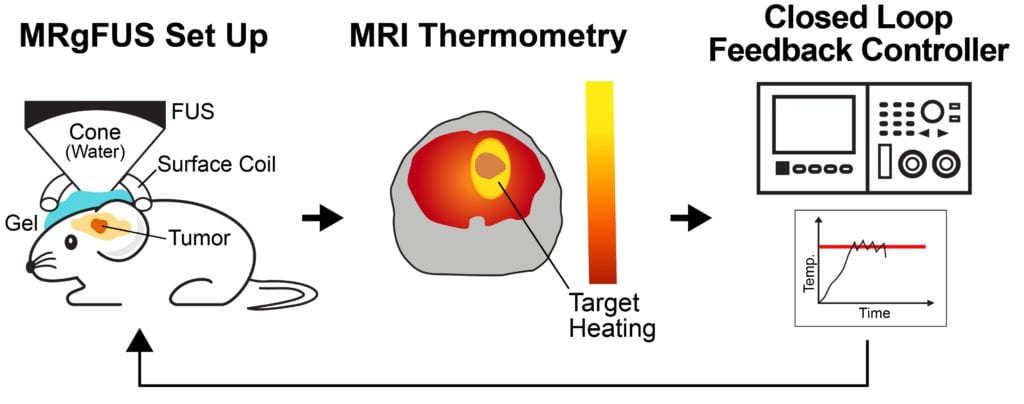
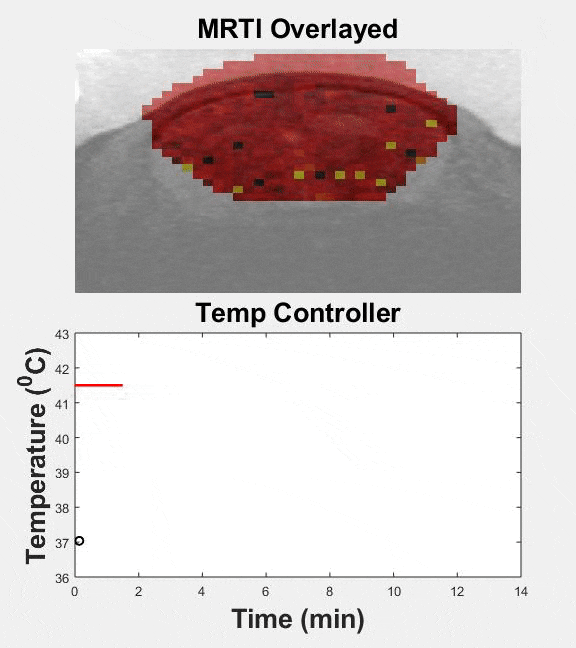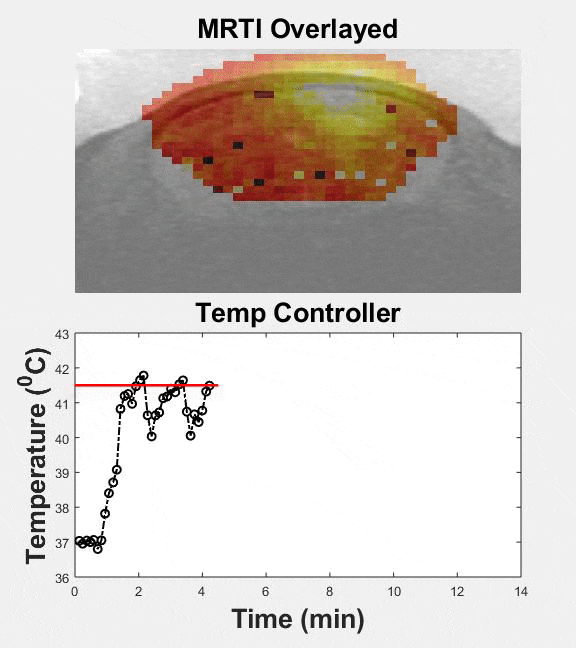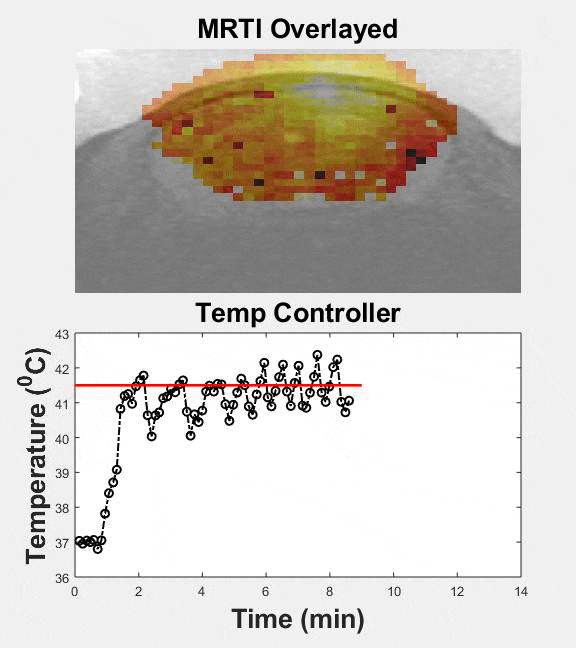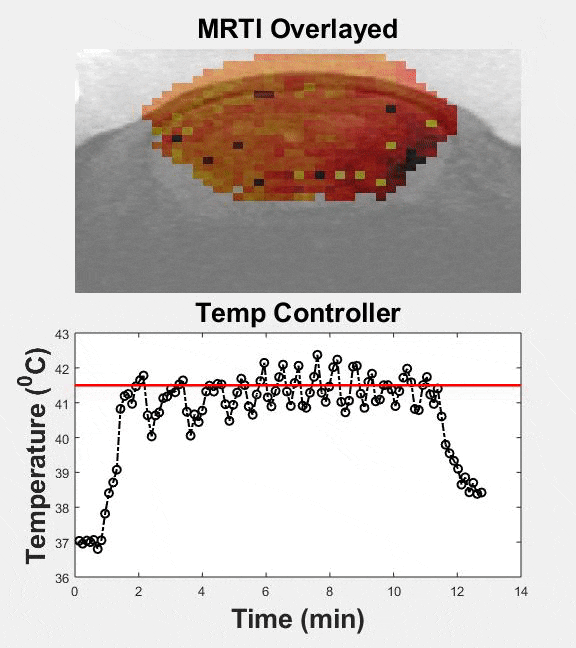
Several passive and active strategies based on different nanoparticle formulations have been proposed for improving drug delivery in brain tumors. Despite improved nanoparticle designs, drug accumulation in brain tumors remains very low. Transcranial MR guided focused ultrasound (MRgFUS) combined with low-temperature sensitive liposomes loaded with doxorubicin (LTSL-Dox ~60nm in radius) may provide a viable therapeutic strategy to improve the penetration of therapeutic agents in brain tumors. We engineered an MRgFUS system with closed-loop methods for controlled transcranial hyperthermia (~12 mins) in order to investigate the release and targeted delivery of doxorubicin in mice brain tumor model. Our investigations indicate that this therapeutic strategy may provide unique opportunities to improve the delivery of NPs and their cargo in the brain tumor microenvironment. Our publication on this topic is currently under review.
 |
 |
|
| Closed-loop TcMRgFUS is able to safely induce hyperthermia in a mouse GL261 intracranial glioma model at 41.5 ⁰C for ~11 minutes | ||
This work is supported by NIH.