Transport barriers in drug delivery represent an ongoing challenge in cancer treatment. While nanomedicine-based anticancer therapies show some promise in overcoming these barriers in the tumor microenvironment and decrease toxicity associated with nonspecific delivery. One of the challenges with the development of localized therapies is the establishment of physiologically relevant assays that model critical features of the tumor microenvironment and allow for a systematic investigation of drug delivery methods and the resulting cellular response, in real time.
To address this need, we have engineered a novel ultrasonic microsystem that combines a microfluidic device that recreates key aspects of the tumor microenvironment with an ultrasound system to study and promote targeted drug delivery and release of therapeutic agents under controlled conditions. Our microsystem, due to vertical integration, can confine in space microbubble-cell interactions, is compatible with optical microscopy, and via integrated temperature and pressure sensors can facilitate controlled (closed-loop) experimentation and mechanistic investigations. The extended functionality of the microsystem was used to demonstrate differential cell response to chemotherapy by establishing drug concentration gradients, using ultrasound-controlled release of chemotherapeutics from temperature sensitive liposomes, and highlighted that DNA damage was confined to the area of drug release. These data demonstrated that it is possible to spatially target the delivery of anticancer agents with ultrasound, while sparing healthy cells.
The proposed in vitro model for focused ultrasound-triggered controlled drug release and response is an important step towards further studies that will quantify release profiles and efficacy of novel ultrasound sensitive nanocarriers and aid in the design of new therapeutic protocols for non-invasive cancer treatments that target tumor cells regionally without damaging adjacent normal cells. Because focused ultrasound based therapies are already in clinical trials for the treatment of a variety of cancer types, the ultrasonic microsystem can also be transformed into a valuable clinical tool, allowing for optimization and personalization of treatment protocol parameters.
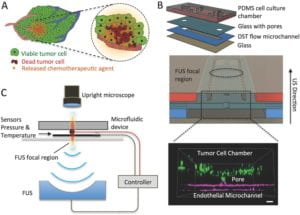 |
Schematic of a closed‐loop acoustofluidic device. A) Schematic showing localized, drug release in a tumor. The area targeted for drug release by FUS is highlighted with the dashed circle. B) Top: assembly of the multilayer microfluidic device. Middle: cross‐section of the microfluidic device showing the cell chamber, DST flow microchannel where the drugs are loaded, and the FUS focal region. Bottom: 3D confocal rendering of GFP tumor cells (green) in the cell chamber and interfaced through the glass pore with the DST flow microchannel where an endothelial monolayer is formed (magenta). C) Diagram of experimental system, highlighting the optically transparent microfluidic device, the pressure/temperature sensors feeding into the controller, and the FUS transducer (located 30 mm away from the device). Small 2016 |